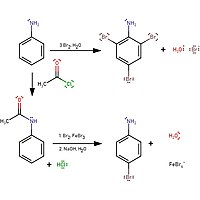
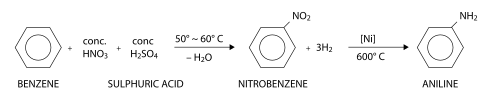
EP0145513A2 - Derivatives of dimethyl-3-nitro-4-amino-aniline, process for their preparation and their use in the dyeing of keratin fibres - Google Patents
Sustainable cycloaliphatic polyurethanes: from synthesis to applications - Chemical Society Reviews (RSC Publishing) DOI:10.1039/D2CS00509C