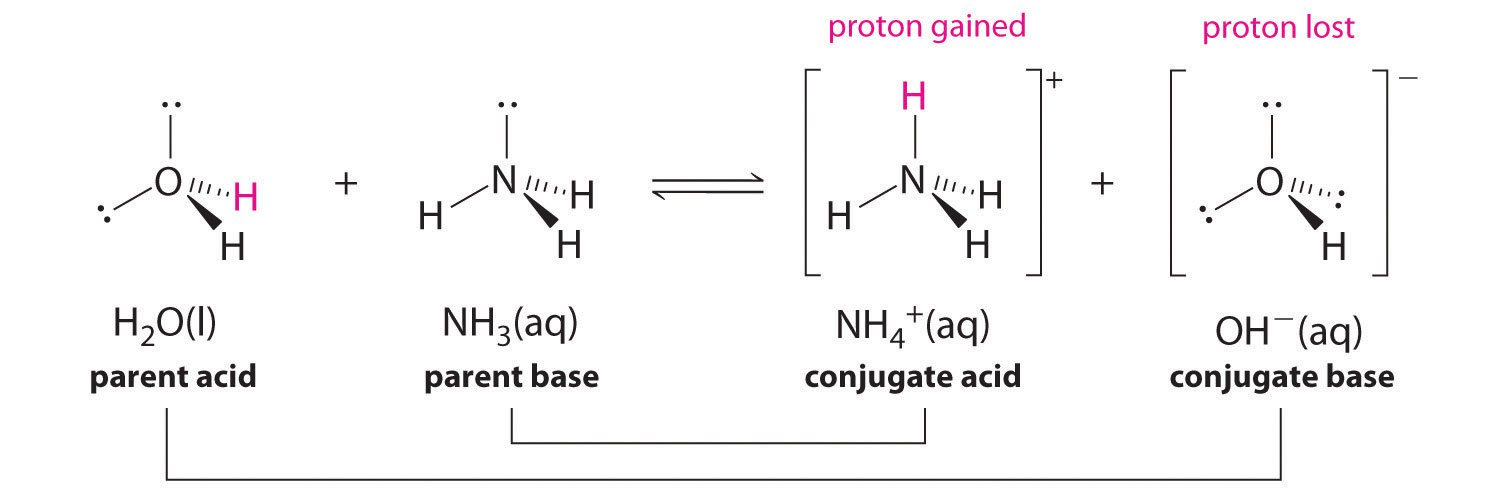
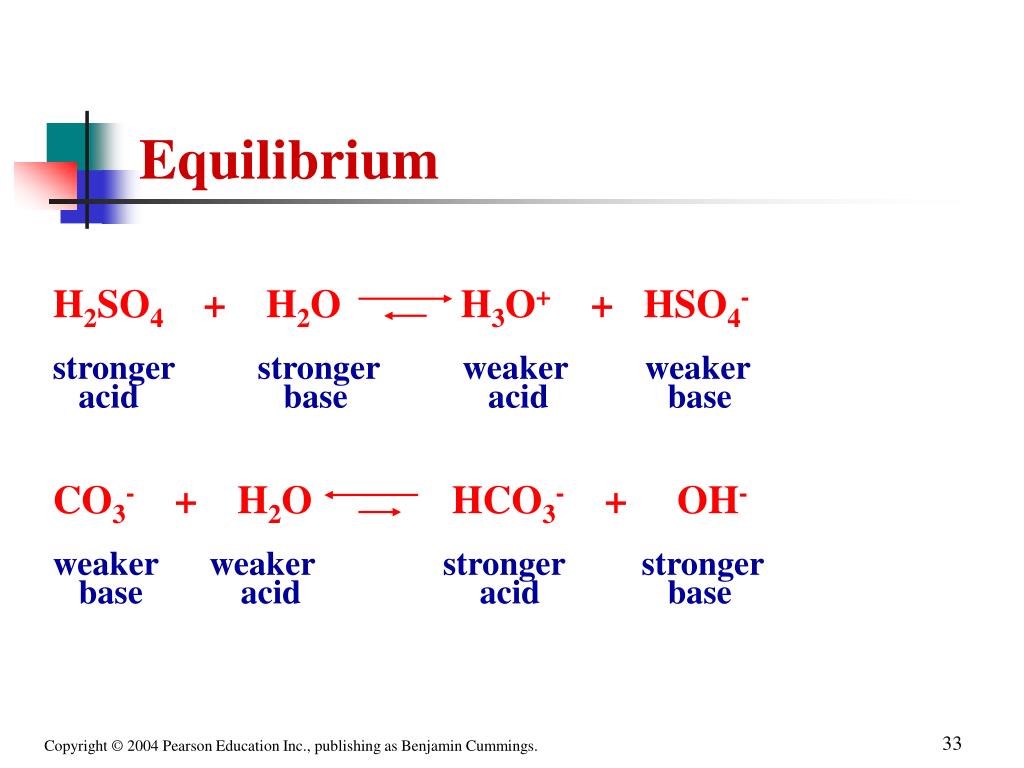
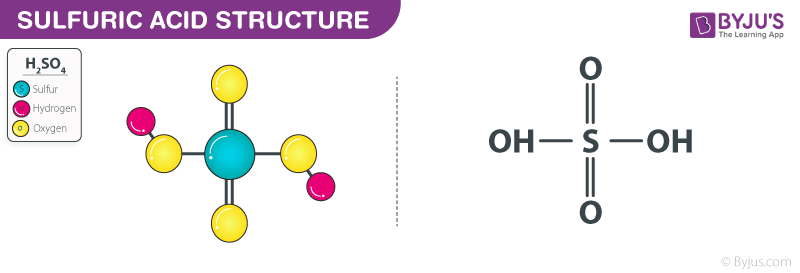
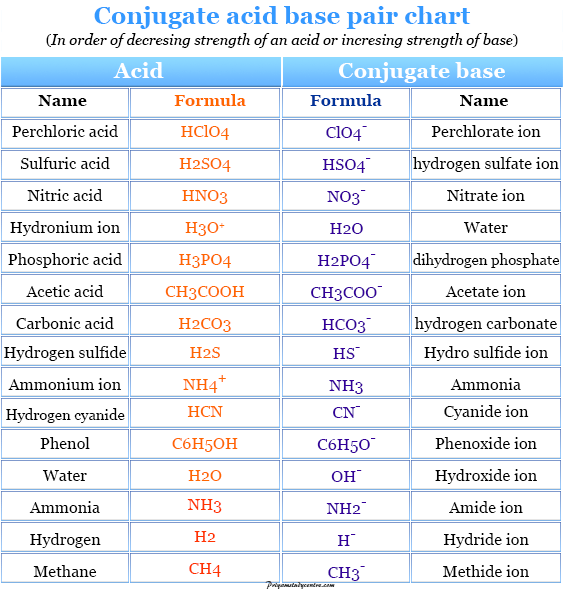
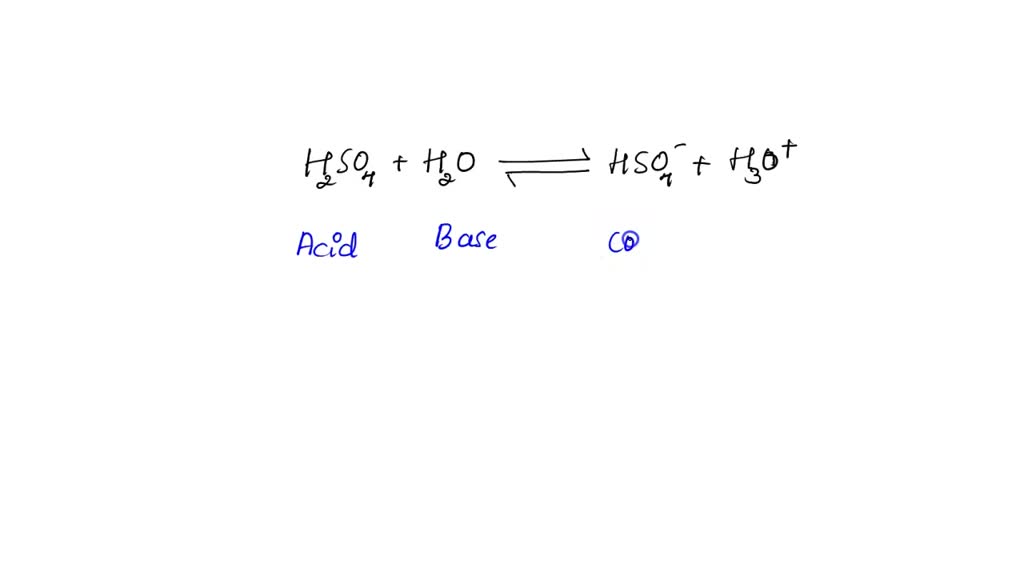Identify the acid-conjugate base pair in this balanced equation: H2SO4 + 2NaOH → 2H2O + - Brainly.com

Identify the conjugate acid-base pairs for the reaction (with the acid written first). CN- + H2O = HCN + OH- |CN- / HCN |HCN / CN- |OH- / H2O |H2O / OH- | Homework.Study.com

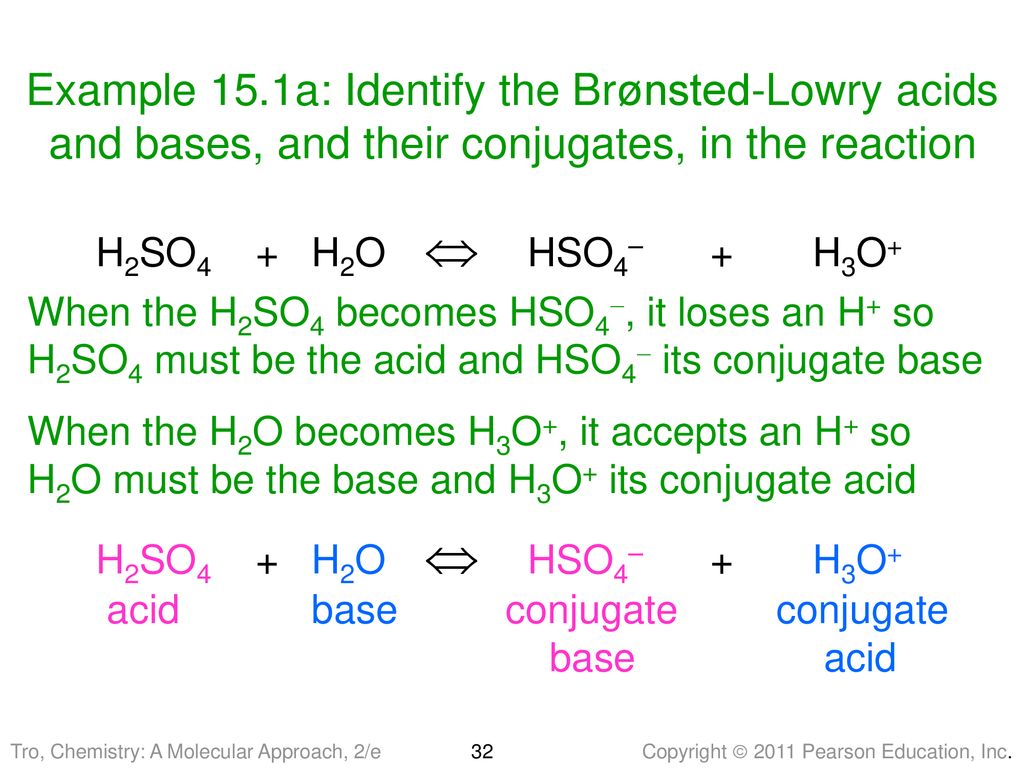
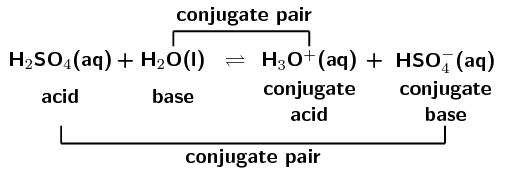
The species: H2O, HCO3^-, HSO4^- and NH3 can act both as Bronsted acids and bases. For each case give the corresponding conjugate acid and base.
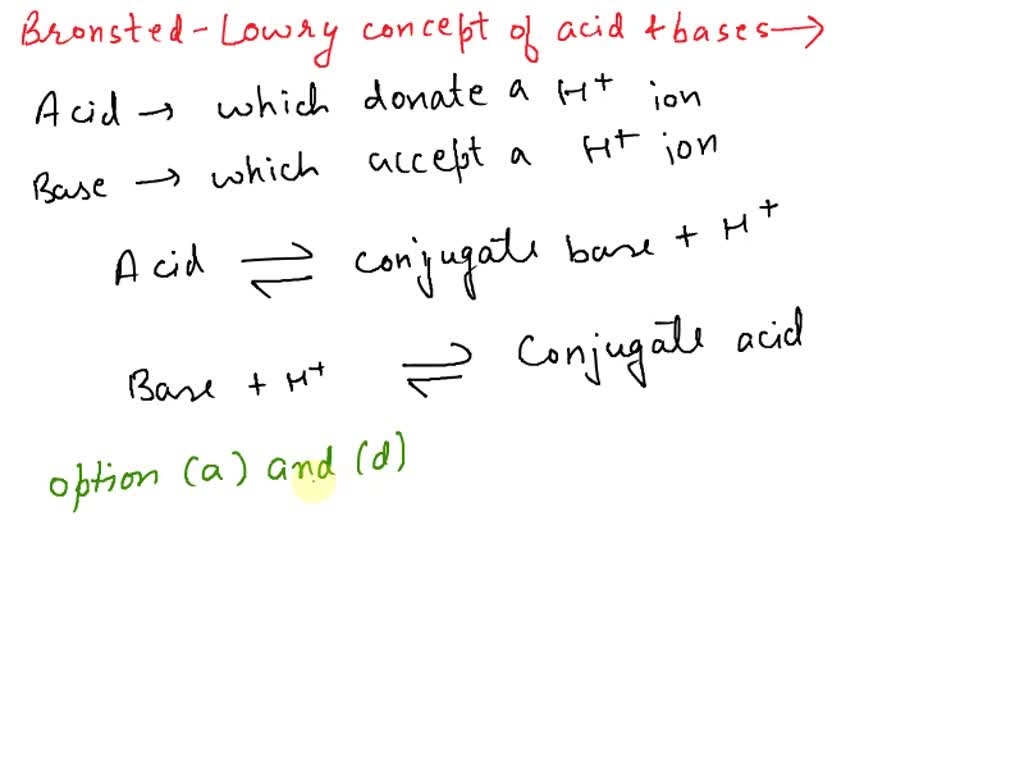
SOLVED: Which of the following represent conjugate acid-base pairs? a. H2O, H3O+ b. OH-, HNO3 c. H2SO4, SO4-2 d. HC2H3O2, C2H3O2-