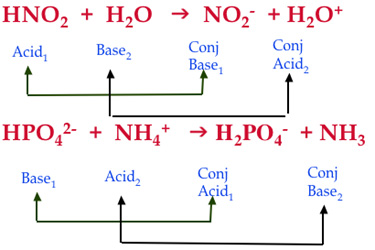
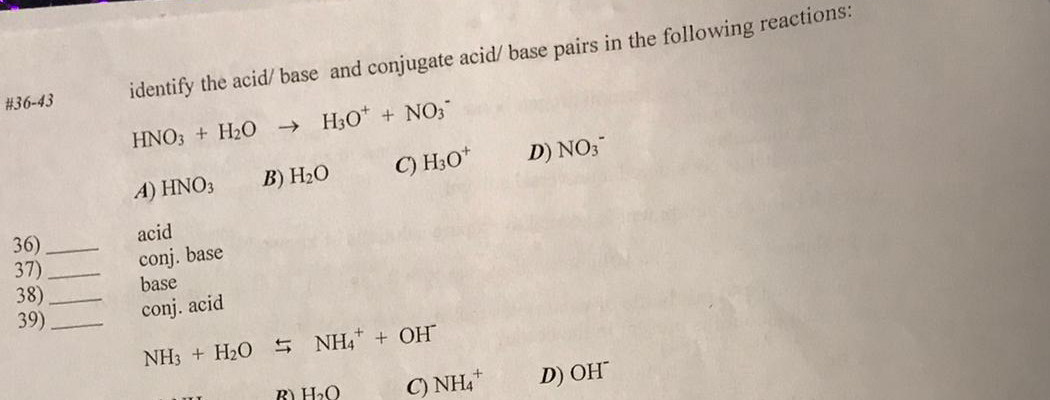Identify the acid, base, conjugate acid, and conjugate base in the following reaction. HNO3 + NH3 + NO3 + - Brainly.com

Nucleation of Mixed Nitric Acid–Water Ice Nanoparticles in Molecular Beams that Starts with a HNO3 Molecule | The Journal of Physical Chemistry Letters

In which of the following pair of reactions first reaction is spontaneous while second reaction is non spontaneous?
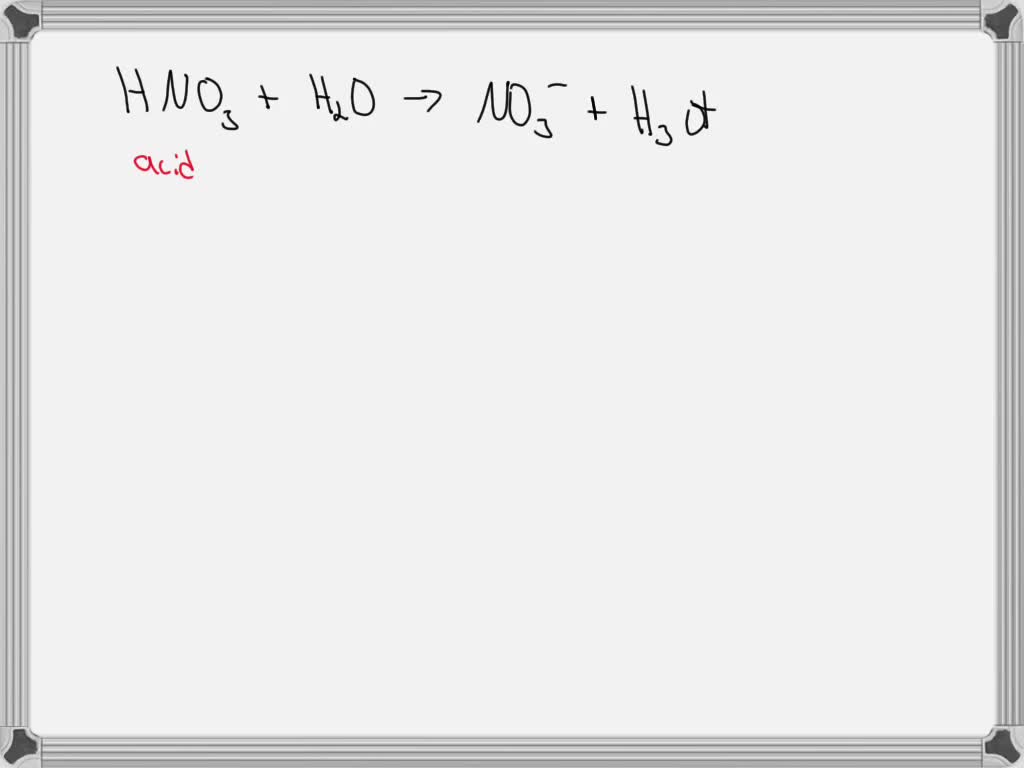
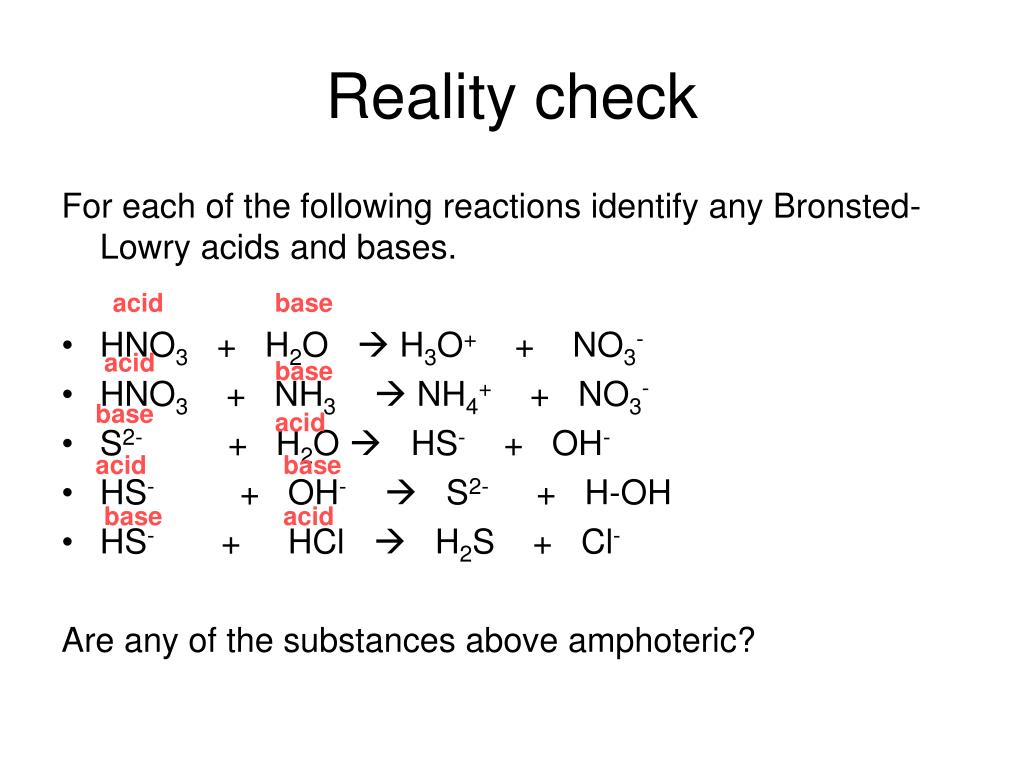
SOLVED: Identify the conjugate acid in the following reaction, HNO3(aq) + H2O(l) → NO3- (aq) + H3O+ (aq). H3O+ NO3- HNO3 H2O None of the above