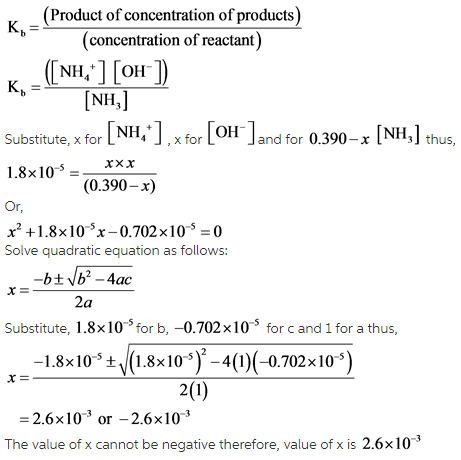
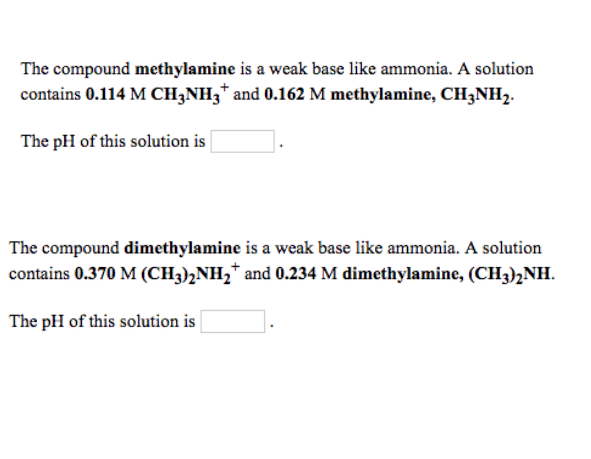
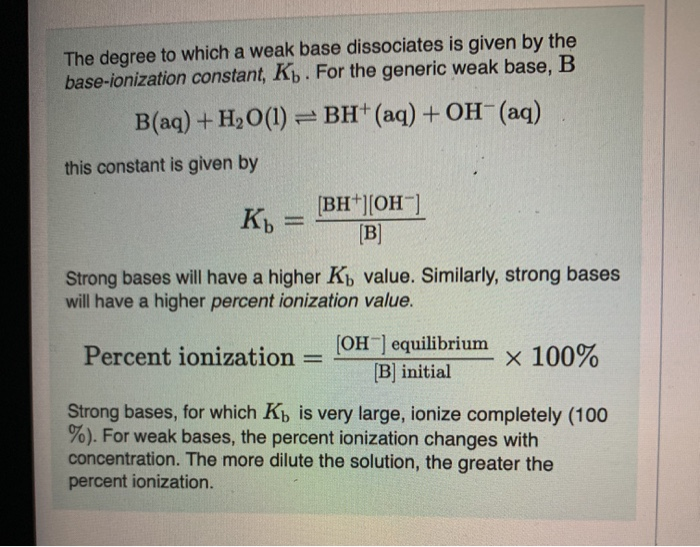
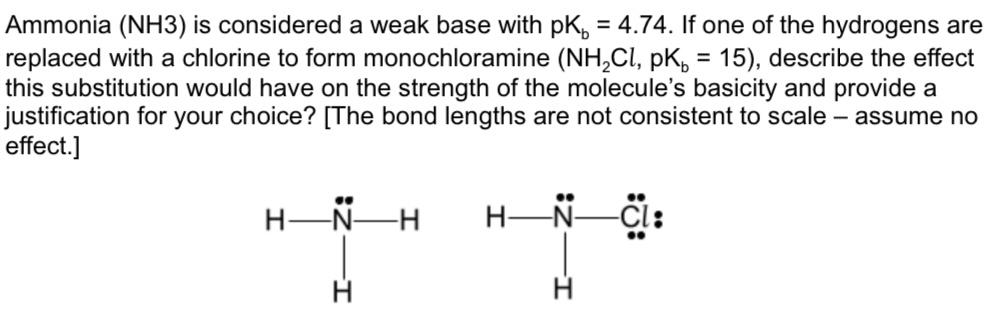
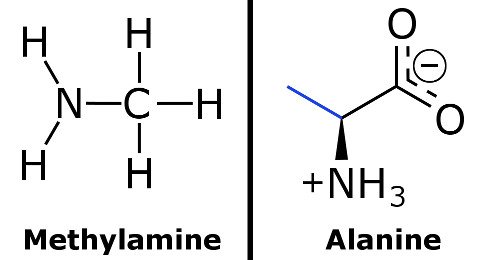
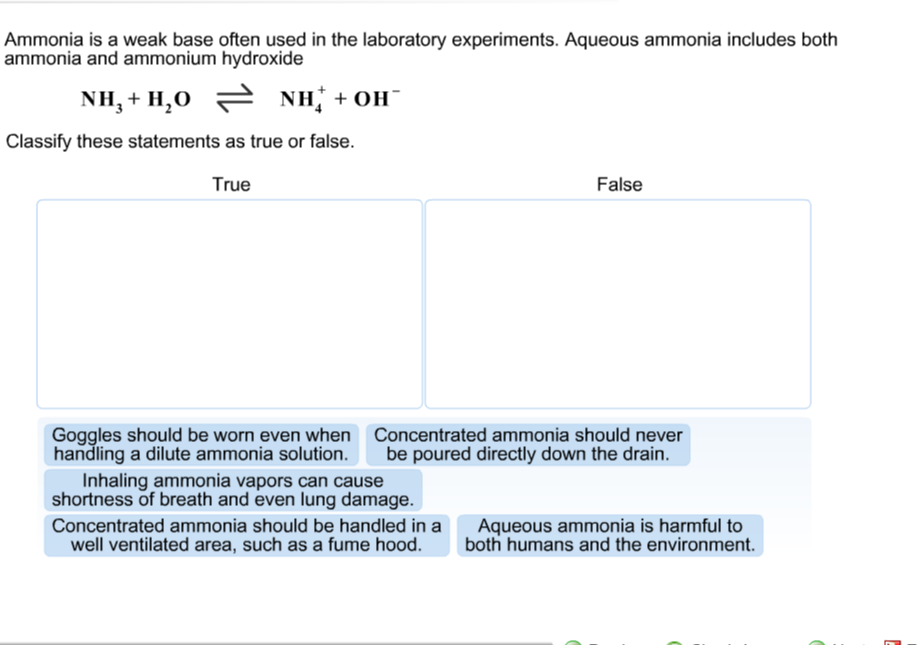
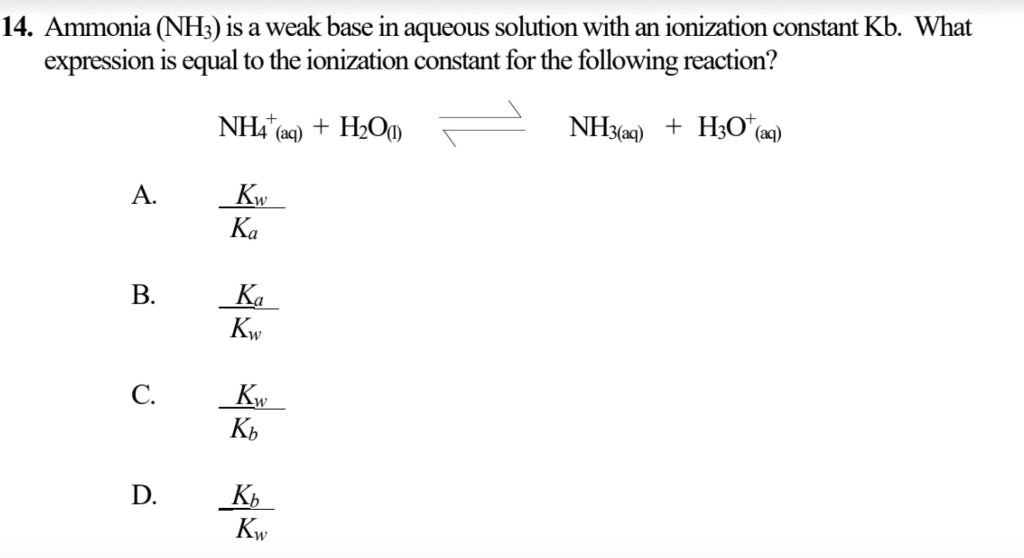
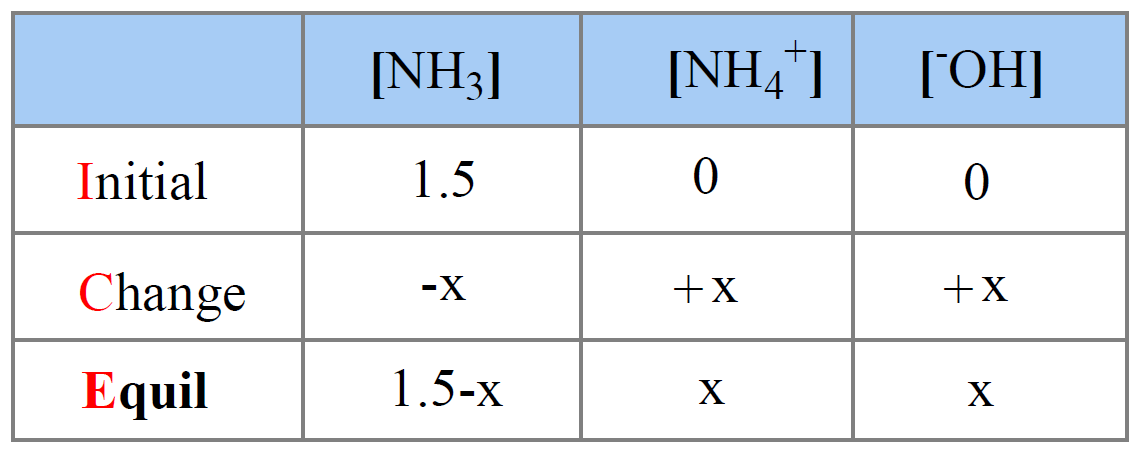
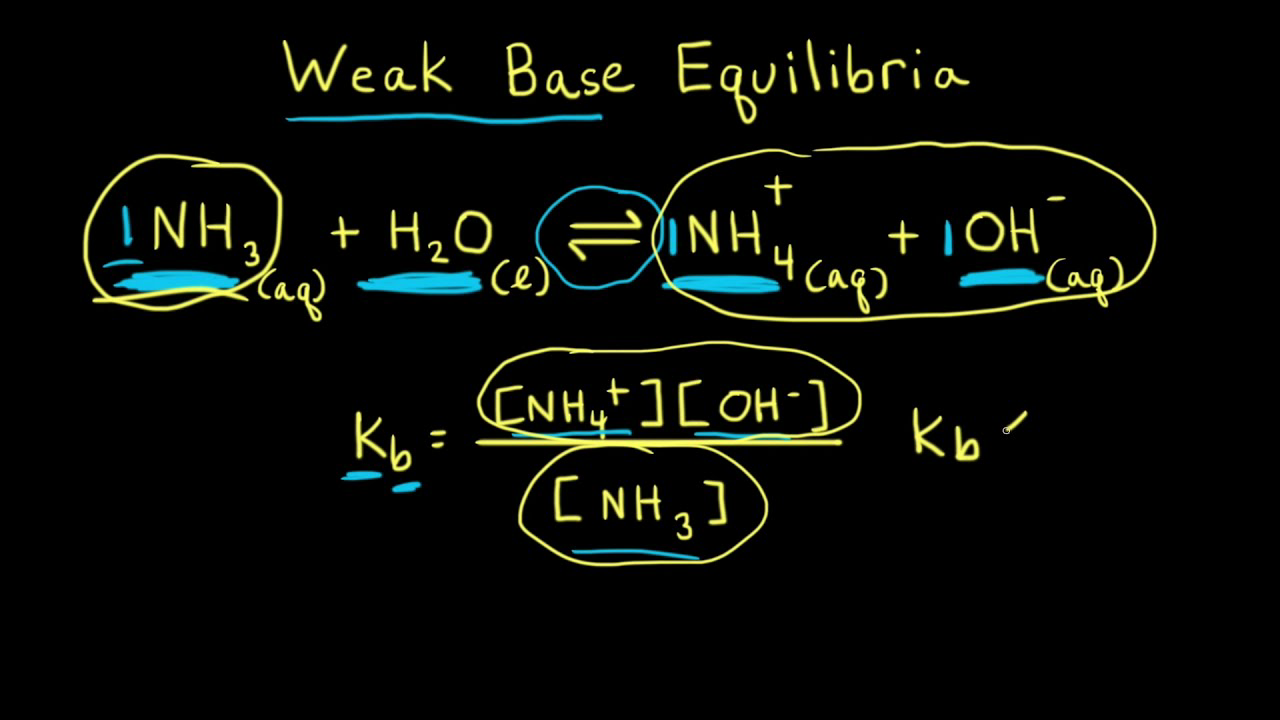Ammonia is the weak base that reacts with water according to the equation: NH3(aq) + H2O(l)⇌NH4^ + (aq) + OH^ - (aq) Will any of the following increase the per cent of
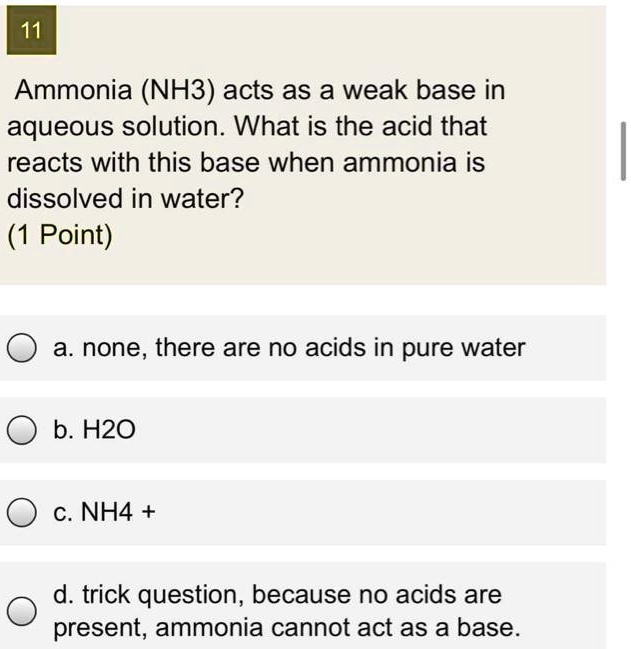
SOLVED: 11 Ammonia (NH3) acts as a weak base in aqueous solution. What is the acid that reacts with this base when ammonia is dissolved in water? (1 Point) none, there are