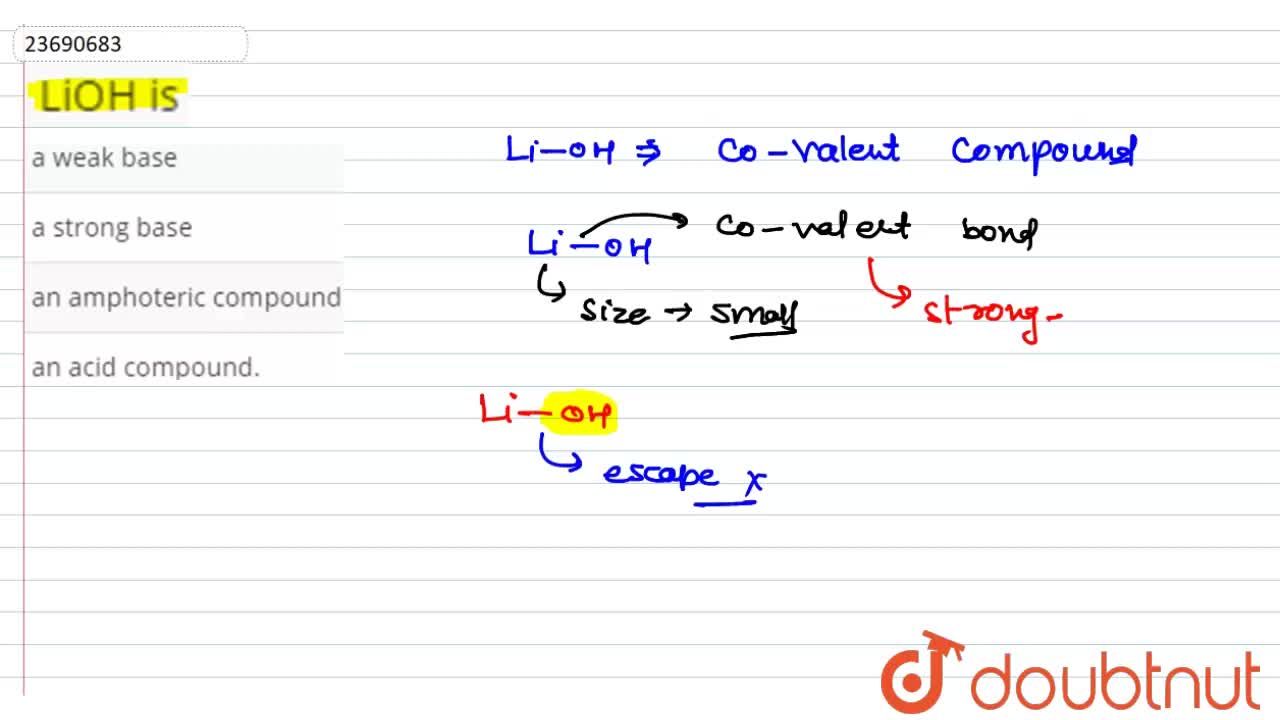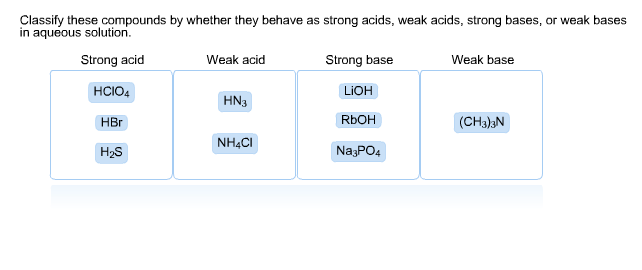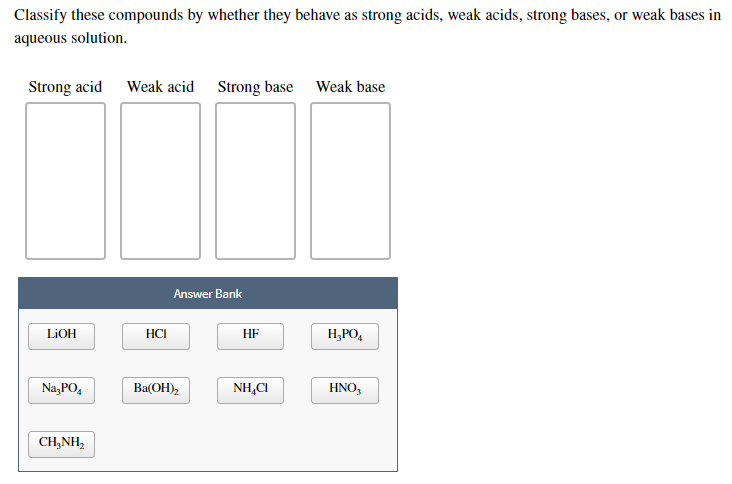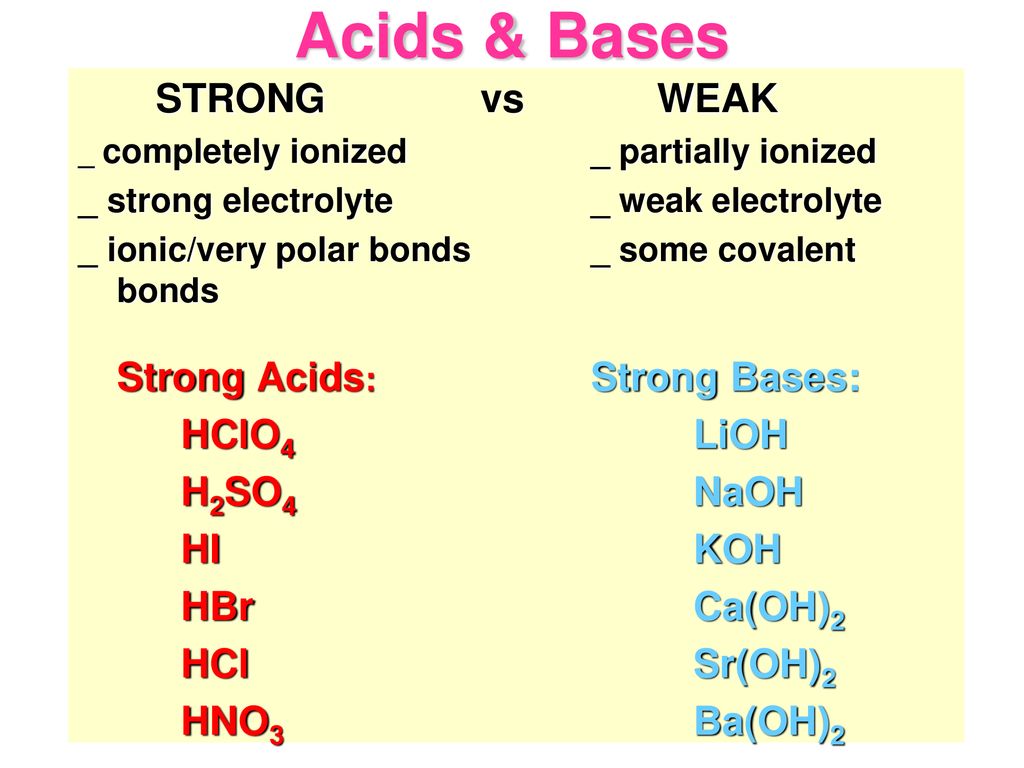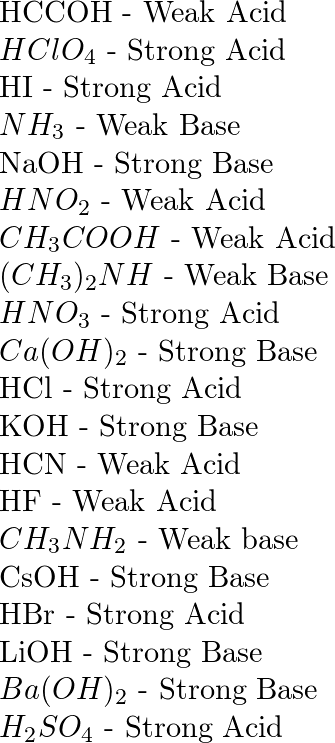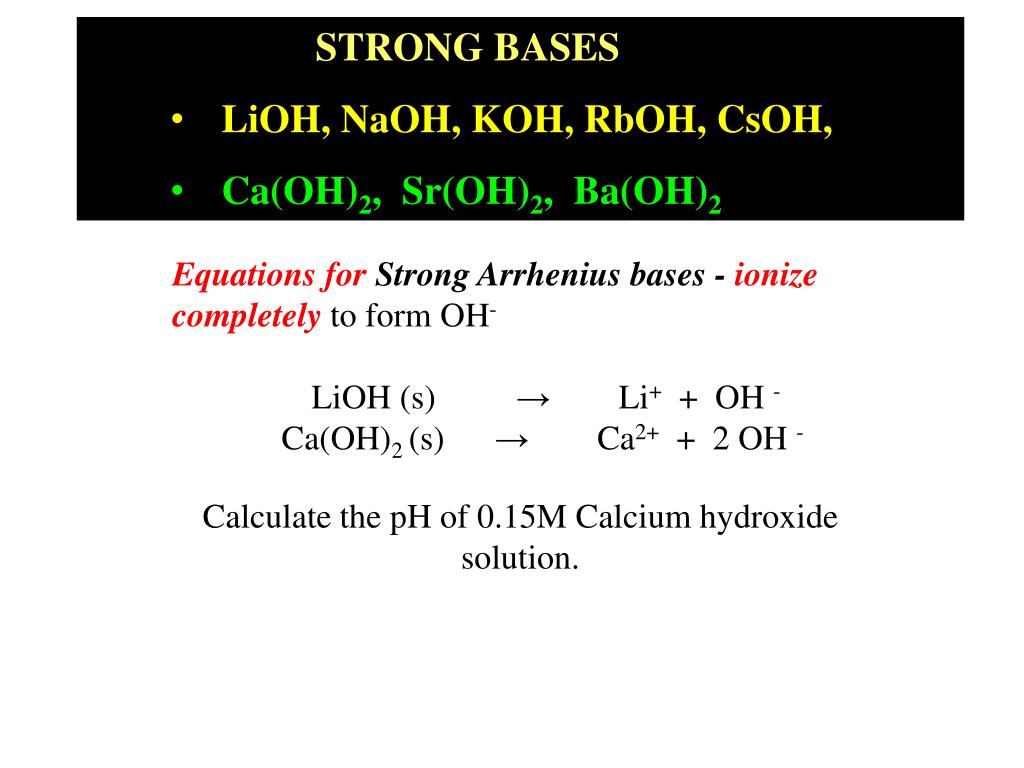
PPT - STRONG BASES LiOH, NaOH, KOH, RbOH, CsOH, Ca(OH) 2 , Sr(OH) 2 , Ba(OH) 2 PowerPoint Presentation - ID:4763546

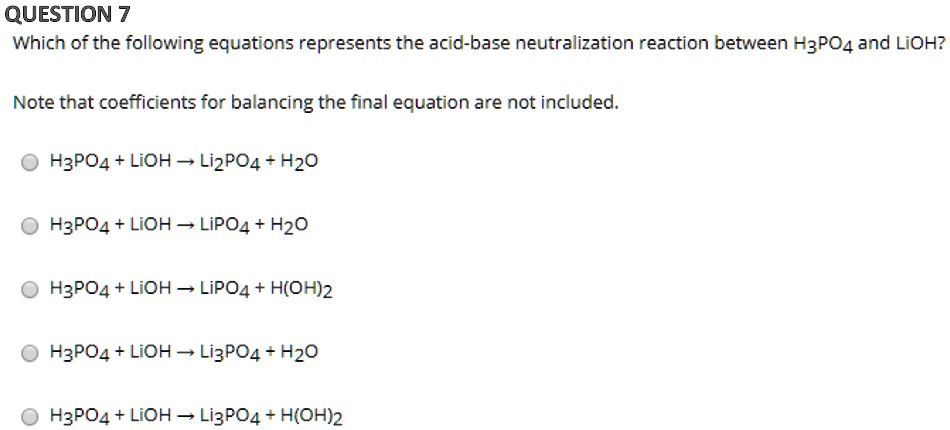
SOLVED:Name each of the following acids or bases: a. H3 PO4 b. LiOH c. HI d. HCN e. HCIO2 f. Ca(OH)2

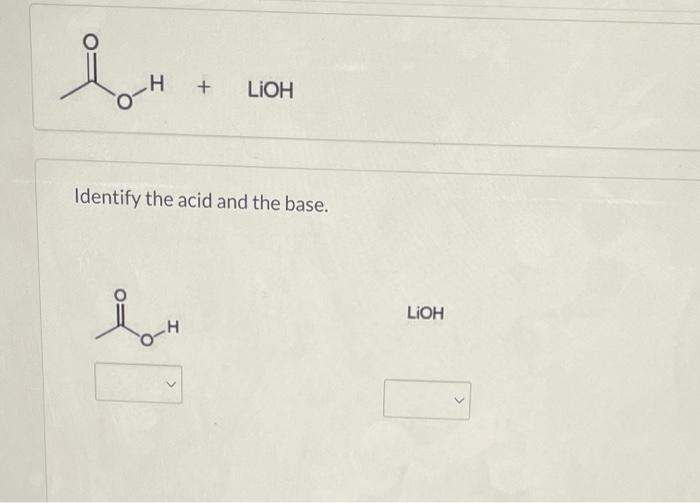
How to Identify the Major Species in a Mixture of Weak and Strong Acids or Bases | Chemistry | Study.com

Titration curves for solutions with 1.0 × 10 −4 moles of LiOH·H 2 O and... | Download Scientific Diagram