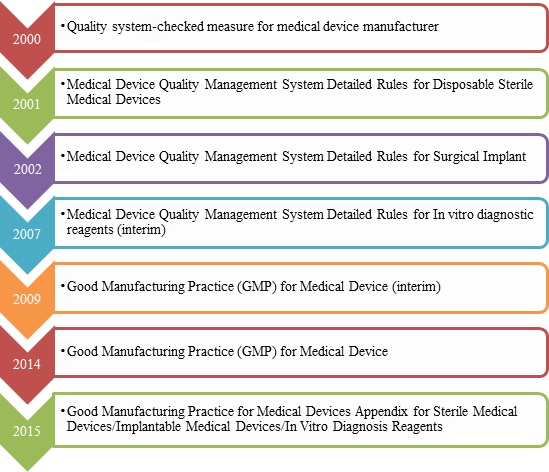
Overview of Good Manufacturing Practice (GMP) for Medical Device - Regulatory News - Medical Devices - CIRS Group

IJERPH | Free Full-Text | Association between the General Practitioner Workforce Crisis and Premature Mortality in Hungary: Cross-Sectional Evaluation of Health Insurance Data from 2006 to 2014
Component Testing Gains Prominence in Drug Product GMP Warning Letters as FDA Focus Intensifies on OTC Topicals and Upstream Sup

Phenotypic variation in yield based selection indices of TOL and GMP... | Download Scientific Diagram

GOOD MANUFACTURING PRACTICES FOR PHARMACEUTICALS 2014/02/181 Faculty of Pharmacy, Omar Al-Mukhtar University, Tobruk, Libya. Dr. Basavaraj K. Nanjwade. - ppt download

Conceptual framework of a food traceability system (based on Aung &... | Download Scientific Diagram

PDF) Untangling the web of European regulations for the preparation of unlicensed radiopharmaceuticals | Clemens Decristoforo - Academia.edu

7/31/2014 Indirect Food Additive GMP Training (Based on the current FDA's Good Manufacturing Practices Guidelines) - ppt download

Book 4C: 2022 Good Manufacturing Practice in the European Union, Refer – Clinical Research Resources, LLC